Russian Federation
Russian Federation
Russian Federation
UDC 639.3
The microalga Isochrysis galbana, due to its nutritional properties, is considered one of important feeds for aquaculture species. Increasing the efficiency of microalgae cultivation with minimal economic costs is the main way to provide live feed and increase profitability of farms. One of the approaches to addressing this issue is the use of phytohormones that have a stimulating effect on the production characteristics and biochemical composition of cultivated microalgae. This study examined the effect of indolyl-3-butyric acid, a phytohormone, at concentrations of 0.1-1 × 10–5 mol. L–1 on the growth of the microalga I. galbana cultivated in an enrichment culture. The duration of the experiment was 7 days. The production characteristics of microalgae were determined by measuring the culture density per 1 mL of the incubation medium as a biomass parameter. Dynamics of cell culture growth were measured using a CytoFLEX flow cytometer. The production characteristics of the culture were also determined on the basis of such parameters as total contents of protein, carbohydrates, lipids, and chlorophyll. The dynamics of the fatty acid composition under the exposure to the phytohormone during cultivation were assessed. The phytohormone at a concentration of 0.4 × 10–5 mol. L–1 was observed to have a stimulating effect on microalgae growth, resulting in a 27% increase over 7 days of cultivation. The effect of indolyl-3-butyric acid at a concentration of 0.4 × 10–5 mol. L–1 on the morphological and biochemical characteristics of the microalgae culture was studied. It was found that the use of the phytohormone resulted in a 57% increase in microalgae cell size compared to the control group. The increase in culture density in the experimental group was accompanied by a 2.6-fold increase in cell aggregates. The increase in protein content after 7 days was 425% in the control group and 538% in the experimental group exposed to the phytohormone. No difference in the carbohydrate content was observed between the control and experimental groups throughout the experiment. Indolyl-3-butyric acid was shown to have a stimulating effect on lipid accumulation. In the exper-imental culture of I. galbana, the total lipid production activity was 1.5-fold higher than in the control culture. An increased contribution of polyunsaturated fatty acids was also noted.
Isochrysis galbana, auxins, microalgae, indolyl-3-butyric acid
Introduction
Scientific interest in cultivation of microalgae is associated with their use as a potential source for the energy, cosmetic, pharmaceutical, and food industries. Microalgae constitute the major food supply for the larval stages of mariculture organisms, including bivalves, crustaceans, echinoderms, etc. [1-4].
In recent years, Isochrysis galbana, which belongs to the class Prymnesiophyceae, has attracted increasing interest due to its nutritional value. Its chemical composition is rich in polyunsaturated fatty acids, including eicosapentaenoic acid (20:5n-3), vitamins B, C, and E, proteins, tocopherols, and pigments such as fucoxanthin, lutein, and β-carotene [5-7].
Isochrysis galbana is characterized by high plasticity to changing environmental factors and a rapid growth rate. Variations in the biochemical composition of this microalga are largely determined by cultivation conditions, including the nutrient medium composition, temperature, salinity, and photoperiod [8]. One of the potential strategies for increasing microalgae biomass production is the use of phytohormones [9].
Phytohormones are small signaling molecules that play a vital role in regulating and coordinating plant growth. They are involved in all developmental processes, including responses to abiotic and biotic stresses [10]. Phytohormones act as external regulators of microalgae resistance to variations in environmental conditions and also influence the biosynthesis of lipids and pigments [11, 12]. The regulatory effect of gibberellic acid on biomass production and metabolite synthesis in Isochrysis galbana is well documented [13, 14]. At low concentrations, phytohormones, such as auxins, promote cell growth by inducing genes responsible cell division. However, at high concentrations, they inhibit growth by acting as herbicides [15]. The mechanisms of action of these hormones are still a subject of ongoing research [16]. Previous studies have shown that auxins increase the growth rate and yield in a number of microalgae species [17]. However, these studies do not always show optimum phytohormone concentrations in terms of biomass and lipid production. The aim of the present study was to determine the effective concentrations of indolyl-3-butyric acid (IBA) and to assess its effect on the growth and biochemical parameters of Isochrysis galbana in an enrichment culture.
Materials and methods
The material for the study consisted of algologically pure cultures of the microalga Isochrysis galbana from the collection of the Far Eastern Technical University of Fisheries. The microalgae were grown in an enrichment mode on an f/2 nutrient medium prepared using filtered and sterilized seawater enriched with mineral salts (NaNO3, NaH2PO4 × H2O, and Na2SiO3 × 9H2O), micronutrients (CuSO4 × 5H2O, ZnSO4 × 7H2O, CoCl2 × 6H2O, MnCl2 × 4H2O, Na2MoO4 × 2H2O, EDTA-Na2, and FeCl3 × 6H2O), and vitamins (В1, В7, and В12) [18]. Microalgae cultures were maintained in an Excella E25 shaker incubator (New Brunswick) at a temperature of 20 ºC an illumination of 9 000 lux, and a light period of 8 h per day. Experimental cultures were grown in 1 000 mL conical flasks with a culture suspension volume of 500 mL. The inoculum was added to the medium at the exponential stage of its growth in a volume of 100 mL. Indolyl-3-butyric acid was used as a growth stimulator at a concentration of 0.1-1.0 × 10–5 mol. L–1 (Hebei Guanlang Biotechnology Co., Ltd., China). Cultures grown without the addition of the growth stimulator were used as the control group. The dynamics of cell culture growth were studied using a CytoFLEX flow cytometer (Beckman Coulter, USA) with a blue laser for excitation (wavelength 488 nm). Data collection and automatic recording were carried out at a constant flow rate of cell suspension through the flow cell (50 μL/min), with the sample collection time limited to 60 s.
The production characteristics of microalgae were determined using the culture density per 1 mL of incubation medium as biomass values [13]. Total carbohydrate content was quantified by acid hydrolysis of samples with the addition of L-tryptophan [19]. Sample preparation for protein assay was performed according to Herbert et al. [20]. Protein content was measured by the Lowry protein assay [21]. Lipid extraction was performed using the Bligh and Dyer method [22]. Total lipid content was measured photometrically by sulfo-phospho-vanillin reaction [23]. Lipid esterification for production of fatty acid methyl esters (FAME) was performed using a freshly prepared acetyl chloride/methanol mixture (1 : 10, v/v) methylation mixture. Fatty acid methyl esters were analyzed on an Agilent 6890 gas chromatograph with a flame ionization detector. An HP Innowax capillary column (30 m × 0.25 mm) was used for separation. The separation parameters were as follows: evaporator temperature of 230 ºC, detector temperature of 240 ºC, and column temperature of
200 ºC (isothermal mode). Helium (He) was used as the carrier gas with a linear flow velocity of 35 cm/s. Identification of monounsaturated fatty acids (MUFA) was carried out by comparing the relative retention times of the FAME sample with standard “carbon number” values based on the calculation of equivalent chain length [24], and by comparing with known standards. Pigment extraction was carried out according to Сarneiro et al. [25]. The quantitative content of chlorophylls was measured spectrophotometrically at wavelengths of 630, 647, 664, and 750 nm. Ninety percent acetone was used as the control [26]. Statistical analysis was performed using the STATISTICA 12.0 program. Data from measurements in at least triplicate were used in the analysis. The data were evaluated by one-way analysis of variance (ANOVA) and a two-tailed Student’s t-test. The significance threshold was p < 0.05.
Results and discussion
The effect of exposure to IBA in the concentration range of 0.1-1 × 10–5 mol. L–1 on the growth dynamics of Isochrysis galbana in an enrichment culture was studied. The study showed a slight stimulating effect on culture growth (15.5%) after 5 days of cultivation only at a phytohormone concentration of 0.4 × 10–5 mol. L–1. The figure shows average culture density values from experiments in triplicate (p ≤ 0.05) (Fig. 1).
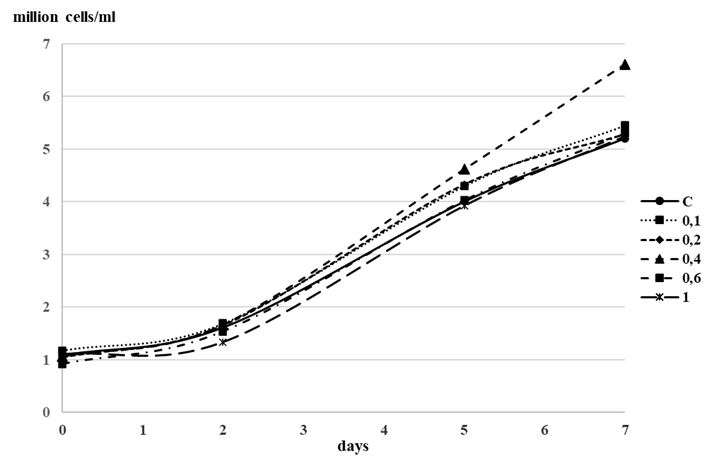
Fig. 1. Effect of different concentrations of indolyl-3-butyric acid (0.1-1.0 × 10–5 mol. L–1)
on the density dynamics of Isochrysis galbana culture: С – control
The effect of IBA on the dynamics of the biochemical composition and morphological characteristics of Isochrysis galbana culture cells was studied at an effective phytohormone concentration of 0.4 × 10–5 mol.
During cultivation, changes in the morphological characteristics of the I. galbana culture were observed. Error whiskers represent standard deviation from the mean value (p ≤ 0.05) (Fig. 2).
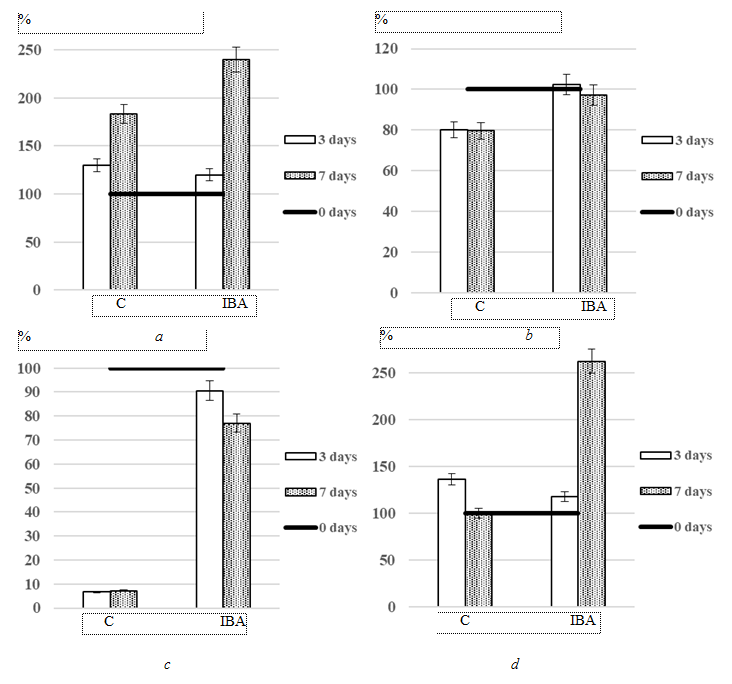
Fig. 2. Effect of indolyl-3-butyric acid (IBA) at a concentration of 0.4 × 10–5 mol L–1 on the morphological characteristics
of Isochrysis galbana culture compared to the control (% of the change in the indicator to the control):
a – size; b – granularity; c – debris; d – aggregates
By day 7 of cultivation, a 57% increase in cell size was recorded for the experimental group compared to the control (Fig. 2, a). The increase in cell size was accompanied by increased granularity of the culture, indicating enlargement of mature microalgal cells (Fig. 2, b). An increase in culture density was accompanied by the formation of cell aggregates. According to the experimental data, the number of Isochrysis galbana cell aggregates in the experimental group treated with IBA was 2.6-fold higher than in the control group (Fig. 2, d). In addition, the results showed a substantial increase in the amount of cell fragments (debris) in the culture exposed to IBA. The values of this parameter in the experimental culture were higher than those in the control 10.7-13.5-fold at different cultivation periods (Fig. 2, c).
The high growth rate of Isochrysis galbana culture under the IBA exposure was apparently accompanied by accelerated maturation of cells and their subsequent apoptosis (Fig. 3: еrror whiskers represent standard deviation from the mean value (p ≤ 0.05)).
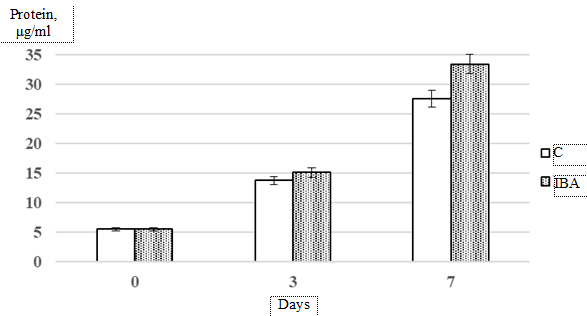
Fig. 3. Dynamics of protein accumulation in Isochrysis galbana culture exposed
to 0.4 × 10–5 mol. L–1 IBA compared to control
The study demonstrated that IBA had a stimulating effect on protein biosynthesis in the Isochrysis galbana culture. Over 7 days of cultivation, the protein concentration increased by 425% in the control group and by 538% in the experimental group.
Carbohydrate synthesis activity is a key characteristic in plant development. On day 3 of cultivation, IBA was found to stimulate carbohydrate accumulation by 15% compared to the control group (Fig. 4: еrror whiskers represent standard deviation from the mean value (p ≤ 0.05)).
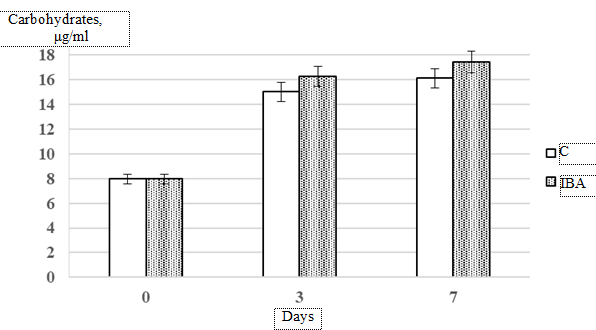
Fig. 4. Dynamics of carbohydrate accumulation in Isochrysis galbana culture exposed
to 0.4 × 10–5 mol. L-1 indolyl-3-butyric acid compared to the control
Lipids are the main energy component of microalgae. The study of lipid dynamics in Isochrysis galbana culture showed that under the IBA exposure the lipid content in the experimental group on day 3 of cultivation was 17.9 μg/mL. Further cultivation did not have an effect on the lipid content in either the experimental or control groups (Fig. 5: error whiskers represent standard deviation from the mean value (p ≤ 0.05)).
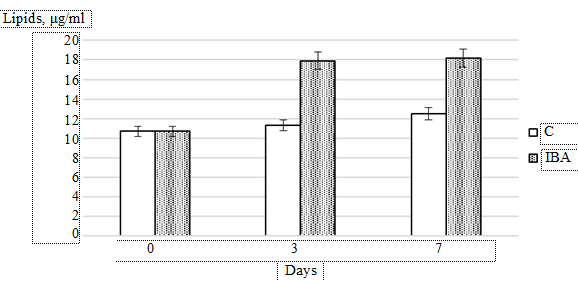
Fig. 5. Dynamics of lipid accumulation in Isochrysis galbana culture exposed
to 0.4 × 10–5 mol. L–1 IBA compared to the control (C)
In the experimental group, lipid accumulation was observed throughout the cultivation period, reaching 12.5 μg/mL on day 7. By the end of the experiment, the difference in lipid content between the experimental and control groups was 46%.
The photosynthetic activity of the Isochrysis galbana culture was assessed on the basis of quantitative chlorophyll content (Fig. 6: error whiskers represent standard deviation from the mean value (p ≤ 0.05)).

Fig. 6. Dynamics of chlorophyll accumulation in Isochrysis galbana culture exposed to 0.4 × 10–5 mol. L–1 IBA
compared to the control
The dynamics of chlorophyll accumulation showed that on day 7 of cultivation, its content in the experimental group was 2.66 μg/mL, which was by 39% higher than in the control group. Over the cultivation period, the total increase in chlorophyll content was 269.2% in the control group and 411.5% in the experimental group (Fig. 6).
Production characteristics of the microalgae were assessed as described by Madani et al. [13], using the culture density per 1 mL of incubation medium as biomass values. Table shows mean values of the parameter under study from experiments in triplicate ± standard error of the mean (p ≤ 0.05) (Table 1).
Table 1
Effect of indolyl-3-butyric acid on the production activity of Isochrysis galbana culture, μg/mL
|
Parameter |
Experimental group* |
Experiment duration, days |
||
|
0 |
3 |
7 |
||
|
Carbohydrates |
C |
0.0855 ± 0.002 |
0.0855 ± 0.003 |
0.31 ± 0.009 |
|
IBA |
0.323 ± 0.009 |
0.385 ± 0.010 |
||
|
Protein |
C |
0.0395 ± 0.001 |
0.324 ± 0.008 |
0.939 ± 0.024 |
|
IBA |
0.317 ± 0.009 |
0.819 ± 0.019 |
||
|
Lipids |
C |
0.0827 ± 0.002 |
0.809 ± 0.018 |
1.06 ± 0.030 |
|
IBA |
0.696 ± 0.021 |
1.42 ± 0.039 |
||
* C – control.
The protein production activities in the experimental and control groups were comparable during the first 3 days of cultivation. However, by day 7, the protein production activity in the control group was higher than in the experimental group. Over the cultivation period, the total protein production activity increased 20.7-fold in the experimental group and 23.8-fold in the control group.
The production activity of lipids in the Isochrysis galbana culture tended to increase throughout the cultivation period. The maximum lipid production activity was observed in the control group, 9.8-fold within the first 3 days of cultivation compared to the initial value. In the experimental group, this value was 8.4-fold. Further cultivation led to a 1.31-fold increase in the control group and a 2.04-fold increase in the experimental group. Overall, the total lipid production activity over the cultivation period increased 12.8-fold in the control group and 17.2-fold in the experimental group compared to the initial value (Table 1).
This study showed the effect of IBA on fatty acid (FA) biosynthesis in microalgae. Table presents mean values of the parameter under study from experiments in triplicate ± standard error of the mean (p ≤ 0.05) (Table 2: fatty acids with percentage contents below 1% are not shown in Table 2, but they were included in the calculation of general parameters).
Table 2
Fatty acid composition of Isochrysis galbana culture, % of total fatty acids
|
Fatty acid* |
Inoculum |
Control |
IBA* |
|||
|
Experimental time, days |
||||||
|
0 |
3 |
7 |
3 |
7 |
||
|
14:0 |
9.80 ± 0.30 |
14.89 ± 0.45 |
14.40 ± 0.40 |
4.02 ± 0.12 |
10.34 ± 0.31 |
|
|
16:0 |
17.86 ± 0.54 |
13.75 ± 0.41 |
12.50 ± 0.39 |
9.36 ± 0.27 |
17.10 ± 0.44 |
|
|
16:1n-9 |
1.98 ± 0.06 |
0.39 ± 0.01 |
4.02 ± 0.12 |
1.15 ± 0.03 |
1.50 ± 0.05 |
|
|
16:1n-7 |
4.63 ± 0.14 |
3.96 ± 0.12 |
0.78 ± 0.03 |
2.17 ± 0.07 |
4.07 ± 0.14 |
|
|
16:4n-3 |
0.62 ± 0.04 |
0.42 ± 0.01 |
0.43 ± 0.02 |
6.79 ± 0.19 |
1.42 ± 0.04 |
|
|
18:0 |
3.64 ± 0.11 |
1.13 ± 0.02 |
0.83 ± 0.03 |
3.36 ± 0.11 |
2.45 ± 0.05 |
|
|
18:1n-9 |
26.05 ± 0.76 |
14.87 ± 0.45 |
13.17 ± 0.37 |
12.05 ± 0.34 |
25.96 ± 0.76 |
|
|
18:1n-7 |
8.94 ± 0.27 |
1.24 ± 0.04 |
1.40 ± 0.04 |
3.62 ± 0.11 |
3.84 ± 0.09 |
|
|
18:2n-6 |
1.99 ± 0.06 |
3.56 ± 0.11 |
3.13 ± 0.08 |
8.92 ± 0.27 |
3.27 ± 0.10 |
|
|
18:3n-3 |
1.89 ± 0.06 |
5.22 ± 0.16 |
5.46 ± 0.16 |
0.87 ± 0.04 |
3.30 ± 0.09 |
|
|
18:4n-3 |
4.24 ± 0.13 |
13.30 ± 0.39 |
15.91 ± 0.44 |
10.36 ± 0.31 |
7.40 ± 0.22 |
|
|
20:1n-11 |
2.10 ± 0.05 |
5.52 ± 0.15 |
6.32 ± 0.18 |
0.98 ± 0.03 |
1.52 ± 0.04 |
|
|
20:2n-6 |
not detected |
6.78 ± 0.19 |
not detected |
|||
|
20:4n-3 |
5.56 ± 0.14 |
|||||
|
20:5n-3 |
1.16 ± 0.04 |
0.59 ± 0.03 |
1.24 ± 0.06 |
not detected |
||
|
22:5n-6 |
2.37 ± 0.06 |
3.02 ± 0.09 |
3.15 ± 0.08 |
5.51 ± 0.17 |
2.61 ± 0.07 |
|
|
22:6n-3 |
6.90 ± 0.19 |
12.35 ± 0.37 |
12.24 ± 0.36 |
3.20 ± 0.10 |
6.91 ± 0.18 |
|
|
SFA |
34.32 ± 1.03 |
32.11 ± 0.92 |
30.03 ± 0.89 |
26.41 ± 0.72 |
35.07 ± 1.01 |
|
|
MUFA |
45.56 ± 1.34 |
28.02 ± 0.83 |
28.02 ± 0.78 |
21.98 ± 0.63 |
39.63 ± 1.14 |
|
|
PUFA |
20.12 ± 0.57 |
39.87 ± 1.12 |
41.96 ± 1.21 |
51.61 ± 1.29 |
25.30 ± 0.67 |
|
* IBA – indolyl-3-butyric acid, 0.4 × 10–5 mol. L–1; SFA – saturated fatty acids; MUFA – monounsaturated fatty acids; PUFA – polyunsaturated fatty acids.
In the control group, a decrease in the concentration of 16 : 0 and 18 : 0 FA was observed during cultivation. The concentration of 14 : 0 FA increased by 4.6%.
In the experimental group exposed to IBA, the total content of saturated fatty acids (SFA) significantly decreased on day 3 of cultivation. However, during this period, the concentration of 16 : 0 FA increased 1.8-fold.
The dynamics of FA composition in the experimental group on day 3 day of cultivation showed a decrease in concentration of MUFA and a twofold increase in concentration of PUFA. Despite the significant increase in PUFA, the synthesis of 20:5n-3 acid by the microalgae culture was inhibited under the IBA exposure. Additionally, the formation of docosahexaenoic acid in the experimental culture was also suppressed.
An interesting aspect of PUFA dynamics was observed in the culture exposed to the phytohormone. On day 3 of cultivation, the accumulation of 20:2n-6 (6.78%) and 20:4n-3 (5.56%) FA was detected in the microalgal cells, which might indicate the activation of an alternative pathway of PUFA biosynthesis under the IBA exposure.
The PUFA composition of the control culture was mainly represented by such FA as 18:3n-3, 18:4n-3, and 22:6n-3, with their concentrations increased multifold by day 7 of cultivation.
Conclusion
The development of an environmentally friendly method for microalgae cultivation is a key factor for scaling up the algae mariculture technology. However, evidence for the actual physiological and growth-promoting roles of auxins in microalgae remains limited. Synthetic auxins such as indoleacetic acid and indolebutyric acid at a concentration of 1 mM have been shown to stimulate increase in culture density by 53 and 46%, respectively.
In our study, IBA had a growth-stimulating effect on the Isochrysis galbana culture within a narrow concentration range. The efficiency of culture density increase reached 27%. The effect of the phytohormone was manifested as an increase in cell size and a decrease in granularity. The rise in culture density led to the formation of cell aggregates and a multifold increase in the number of destroyed cells (debris).
This study showed that the IBA exposure had a stimulating effect on the synthesis of proteins, carbohydrates, and lipids in microalgae in the enrichment culture. An increased carbohydrate productivity was observed on day 3, and an increased lipid productivity was observed on day 7 of cultivation. Furthermore, there was a direct correlation between the chlorophyll concentration and the lipid synthesis under the IBA exposure.
Indolyl-3-butyric acid had little effect on the FA composition of Isochrysis galbana lipids. The general trend in the FA composition was characterized by
a decrease in the MUFA content and an increase in PUFA levels.
Thus, the conducted study has demonstrated feasibility of modulating the composition of Isochrysis galbana components under the effect of IBA.
1. Koyande A. K., Chew K. W., Rambabu K., Tao Y., Chu D. T., Show P. L. Microalgae: A potential alternative to health supplementation for humans. Food Science and Human Wellness, 2019, vol. 8, pp. 16-24.
2. Rastar M., Pezhman S., Hosseini Shekarabi S. P., Mehdi S., Mehrgan Sabzi S. Effects of iron and zinc concentrations on growth performance and biochemical composition of Haematococcus pluvialis: a comparison between nanoparticles and their corresponding metals bulks. Journal of Algal Biomass Utilization, 2018, vol. 9, no. 2, pp. 59-67.
3. Rizwan M., Mujtaba G., Memon S. A., Lee K., Rashid N. Exploring the potential of microalgae for new biotechnology applications and beyond: a review. Renewable Sustainable Energy Reviews, 2018, vol. 92, pp. 394-404.
4. Zhao Y., Wang H. P., Han B., Yu X. Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: a review. Bioresource Technology, 2019, vol. 274C, pp. 549-556.
5. Matos J., Cardoso C., Bandarra N., Afonso C. Microalgae as a healthy ingredient for functional food: A review. Food & Function, 2017, vol. 8, pp. 2672-2685.
6. Bandarra N., Pereira P., Batista I., Vilela M. Fatty acids, sterols and α-tocopherol in Isochrysis galbana. Journal of Food Lipids, 2003, vol. 10, no. 1, pp. 25-34.
7. Raposo M. F., Morais R. M. S. C., Morais A. M. M. B. Health applications of bioactive compounds from marine microalgae. Life Sciences, 2013, vol. 93, no. 15, pp. 479-486.
8. Sadovskaya I., Souissi A., Souissi S., Grard T., Lencel P., Greene C. M., Dmitrenok P. S., Chizhov A. O., Shashkov A., Usov A. I. Chemical structure and biological activity of a highly branched (1 → 3,1 → 6)-β-D-glucan from Isochrysis galbana. Carbohydrate Polymers, 2014, vol. 111, no. 11, pp. 139-148.
9. Fierli D., Aranyos A., Barone M. E., Parkes R., Touzet N. Influence of exogenous phytohormone supple-mentation on the pigment and fatty acid content of three marine diatoms. Applied Microbiology and Biotechnology, 2022, vol. 106, pp. 6195-6207.
10. Spoel S. H., Dong X. Making sense of hormone crosstalk during plant immune responses. Cell Host and Microbe, 2008, vol. 3, pp. 348-351.
11. Romanenko E. A., Kosakovskaia I. V., Romanenko P. A. Fitogormony mikrovodoroslei: biologicheskaia rol' i uchastie v reguliatsii fiziologicheskikh protsessov. Chast' II. Tsitokininy i gibberelliny. Al'gologiia, 2016, vol. 26, no. 2, pp. 203-229.
12. Priyadarshani I., Rath B. Commercial and industrial applications of microalgae – A review. Journal of Algal Biomass Utilization, 2012, vol. 3, no. 4, pp. 89-100.
13. Madani N. S. H., Shekarabi S. P. H., Mehrgan M. S., Pourang N. Can 2, 4-dichlorophenoxyacetic acid alter growth performance, biochemical composition, and fatty acid profile of the marine microalga Isochrysis galbana? Phycologia, 2020, vol. 59, no. 6, pp. 1-8.
14. Leskova S. E., Mikheev E. V., Kovalev N. N. Rost Isochrysis galbana v miksotrofnykh usloviiakh s ispol'zovaniem gibberellinovoi kisloty. Journal of Agriculture and Environment, 2022, vol. 22, no. 2, pp. 1-6.
15. Grossmann K. Auxin herbicide action. Plant Signaling & Behavior, 2007, vol. 2, no. 5, pp. 421-423.
16. Lau S., Jurgens G., De Smet I. The envolving com-plexity of the auxin pathway. Plant Cell., 2008, vol. 20, pp. 1738-1746.
17. Salama E.-S., Kabra A. N., Ji M.-K., Kim J. R., Min B., Jeon B.-H. Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresource Technology, 2014, vol. 172, pp. 97-103.
18. Guillard R. R. L. Culture of Phytoplankton for Feeding Marine Invertebrates. Culture of Marine Invertebrates Animals. New York, Plenum Press, 1975. Pp. 29-60.
19. Laurens L. M. L., Dempster T. A., Jones H. D. T., Wolfrum E. J., Wychen S. V., McAllister J. S. P., Rencen-berger M., Parchert K. J., Gloe L. M. Algal Biomass Con-stituent Analysis: Method Uncertainties and Investigation of the Underlying Measuring Chemistries. Journal of Analytical Chemistry, 2012, vol. 84, no. 4, pp. 1879-1887.
20. Herbert D., Phipps P. J., Strange R. E. Chemical analysis of microbial cells. Methods in Microbiology, 1971, no. 5, pp. 209-344.
21. Lowry O., Rosenbrougt N., Parr A., Randall R. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry, 1951, vol. 193, no. 1, pp. 265-276.
22. Bligh E. G., Dyer W. I. A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 1959, vol. 37, pp. 911-918.
23. Johnson K. R., Ellis G., Toothill C. The sulfophos-phovanilin reaction for serum lipids: a reappraisal. Clinical Chemistry, 1977, vol. 23, no. 9, pp. 1669-1678.
24. Christie W. W. Lipid analysis: Isolation, identifica-tion and structural analysis of lipids. Bridgwater, England, The Oily Press, 2003. 416 p.
25. Carneiro M., Pojo V., Malcata F. X., Otero A. Lipid accumulation in selected Tetraselmis strains. Journal of Applied Phycology, 2019, vol. 31, no. 5, pp. 2845-2853.
26. Aminot A., Ray F. Chlorophyll a: Determination by spectroscopic methods. ICES techniques in marine environmental sciences, 2001, no. 30, 17 p.